By VCU Massey Cancer Center
In pre-clinical studies, VCU Massey Cancer Center scientists have developed a novel drug delivery system using nanoparticles that significantly outperformed the tumor-targeting abilities of its predecessors. The researchers are hopeful their findings could lead to improved outcomes and reduced side effects for cancer patients undergoing chemotherapy.
Drug delivery systems are engineered technologies designed to control the rate at which a drug is released and the location in the body where it is released. Previous scientific efforts have been committed to developing tumor-targeted drug delivery systems; however, patterns of inadequate blood circulation and low targeting specificity have negatively impacted therapeutic outcomes.
To combat these setbacks, a team of researchers led by Hu Yang, Ph.D., a professor in the Department of Chemical and Life Science Engineering at the VCU College of Engineering and a member of the Developmental Therapeutics research program at Massey, designed a new drug delivery platform called leutusome that is a composite of nanoparticles from both leukocytes (white blood cells) and tumor cells.
“Compared to previously researched and reported drug delivery systems, leutusome represents the most advanced delivery system with the highest tumor-targeting ability,” said Yang, who is also a professor in the Department of Pharmaceutics at the VCU School of Pharmacy.
Leutusome is referred to as a cell-membrane-camouflaged biomimetic nanoplatform, meaning that it is a composite cellular nanoparticle that mimics the properties of multiple other cell types. Think of leutusome as an undercover, miniaturized cellular vehicle whose properties of both immune cells and cancer cells create a sort of disguise, or “membrane camouflage,” that allows it to travel through cellular systems undetected and perform functions that conventional nanoparticles would not be able to do.
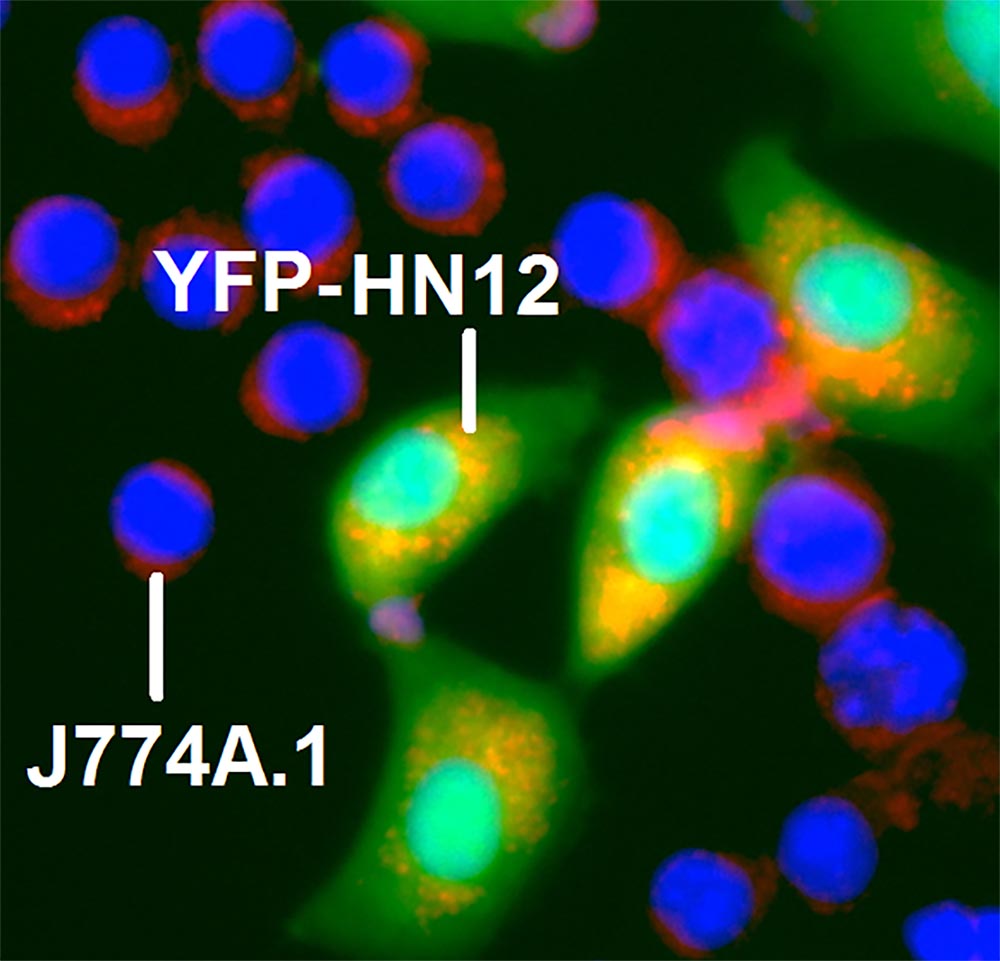
The properties of leukocytes, white blood cells pivotal to immune response, led to significantly prolonged blood circulation, while the properties of cancer cells enhanced the drug’s ability to identify and target tumor cells in a mouse model of head and neck cancer.
“Empowered with a high drug-loading capacity, the composite leukocyte and tumor cell membrane has proven more efficient than a tumor cell membrane alone in directing camouflaged particles to the tumor,” Yang said.
Yang fused leutusome with the chemotherapy drug paclitaxel before injecting it into the cancer mouse model. Paclitaxel is an FDA-approved drug sold under the brand name Taxol, among others, and is used to treat a variety of different cancers.
“Using our drug delivery system, paclitaxel was found to most potently inhibit tumor growth while not causing systemic adverse effects,” Yang said.
The research, published in Nano Letters, yielded leutusome levels in the tumor of approximately 80 percent injected dose per gram of tissue (ID/g), 1.7-fold greater than previous drug delivery systems. A recent meta-analysis surveying literature from the past 10 years revealed that tumor accumulation of nanoparticles in rodent models through active targeting ranged from 0.02 to 45.8 percent ID/g with an average of 4.6 percent ID/g.
“The accumulation of leutusome in the tumor reached nearly two times higher than any other nanoparticles reported in previous literature,” Yang said. “We will continue to examine this platform for its capabilities in cancer immunotherapies and test the feasibility of using tumor cells from patients to develop personalized cancer nanomedicine.”
While exciting and promising, the research is too early at this stage for testing in clinical trials. Yang and his collaborator Xiang-Yang Wang, Ph.D., a member of the Cancer Molecular Genetics research program at Massey Cancer Center, are seeking NIH grant funding to continue the system’s application in immunotherapy, with the eventual goal to test the system in patients.
Yang and Wang collaborated on this research with Hongliang He, Ph.D., from the VCU Department of Chemical and Life Science Engineering; Shobha Ghosh, Ph.D., and Jing Wang, from the VCU Department of Internal Medicine; Chunqing Guo, Ph.D., instructor in the VCU Department of Human and Molecular Genetics; and William Korzun, Ph.D., an associate professor at the VCU College of Health Professions.
